Accola offers flexible contract manufacturing services to meet changing production demands, reduce lead times, and support growth at scale. We support everything from small batch builds to full-scale commercial production for drug delivery systems, medical devices, and diagnostics.

Injection Molding
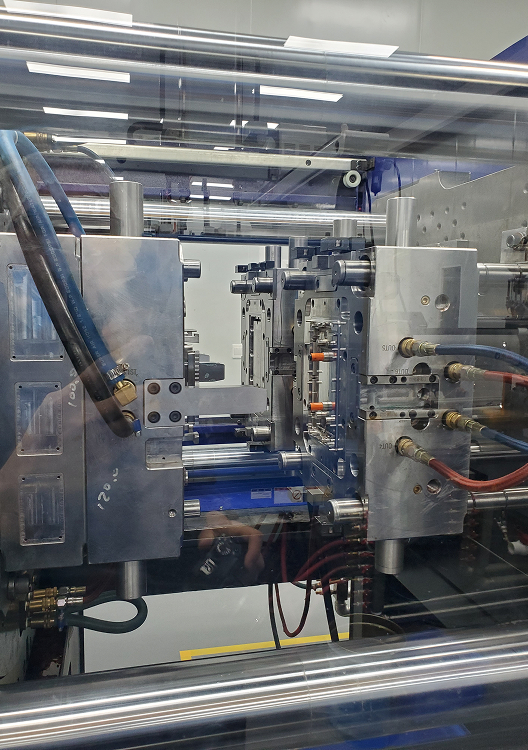
We offer in-house cleanroom and white room injection molding services to produce components used in medical and drug delivery systems. Our team excels at designing parts that transition seamlessly to molding tools, ensuring manufacturability and reliability.

We manufacture complex, multi-material components with tight tolerances and high repeatability using fully electric thermoplastic systems and up to 64-cavity molds.

Welding and bonding options such as ultrasonic, RF, and solvent bonding are available for complex builds.
Drug Handling
Our facilities are capable of manufacturing drug-device combination products like auto-injectors, cartridges, and pre-filled syringes. Assembly and packaging take place in ISO Class 7 and 8 cleanrooms under an ISO 13485-certified quality system that meets FDA, cGMP, and international combination product regulations.

Our packaging equipment enables sterile sealing and tamper-evident features to support safe drug preparation and delivery.

We provide drug handling services for small batches and clinical trials, enabling the initial market release of new combination products.


Sterile Barrier Packaging
Sterile barrier packaging is performed using validated processes such as pouch sealing, tray sealing, kitting, and labeling. Formats are selected based on sterility needs and regulatory classification, with in-process controls and final inspection to ensure compliance.

Packaging workflows are documented to meet regulatory requirements and maintain product safety.

Barrier systems are chosen based on sterility needs to protect product integrity during clinical and commercial use.
Design Transfer
Design transfer is structured to support manufacturing, validation, and long-term scale-up. Cross-functional teams collaborate with product engineers early to align on tooling, automation, and compliance, following defined checkpoints and maintaining full documentation for traceability and regulatory readiness.

We develop documentation in parallel with the build process to support both regulatory submissions and manufacturing needs.

We design transfer plans to streamline validation and ensure a smooth transition into production.